Chemistry, 23.04.2020 01:44 Fireburntbudder
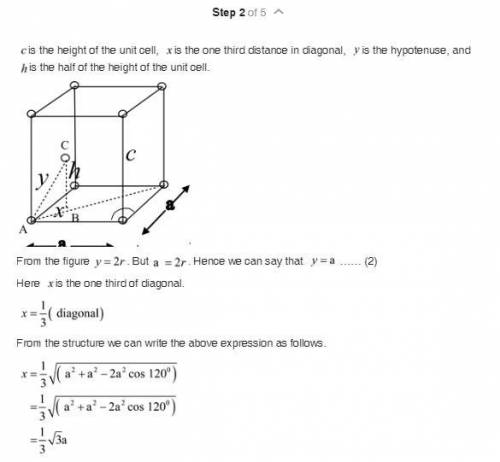
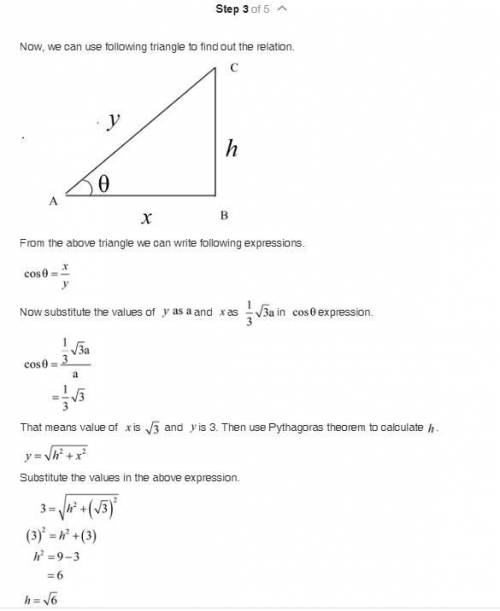
Show that the ideal c/a ratio (height of unit cell divided by edge length) for the HCP unit cell is 1.633. (You may wish to refer to Exercise E.2 in the text page GL 1-6) Comment on the fact that real HCP metals display c/a ratios varying from 1.58 (for Be) to 1.89 (Cd). The atomic radius of HCP Mg is 0.1605 nm. Find the lattice constants, c and a, the c/a ratio and theoretical density for Mg.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
10. translate each of the following chemical equations into a sentence. a. 2 zns(s) + 3 o2(g) -> 2 zno(s) + 2 so2(g) b. cah2(s) + 2 h2o(l) -> ca(oh)2 (aq) + 2 h2(g)
Answers: 2


Chemistry, 22.06.2019 04:00
You encounter a solution that is acidic and you decide to test it by adding a small amount of a strong acid. the ph lowers slightly but is approximately unchanged, and still remains acidic. what can you say about the solution? a. it is a buffer solution. b. it is not a buffer solution it is a strong acid solution. d. the solution has been neutralized. e. the solution has excess acid present
Answers: 1

Chemistry, 22.06.2019 07:20
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1
You know the right answer?
Show that the ideal c/a ratio (height of unit cell divided by edge length) for the HCP unit cell is...
Questions

Mathematics, 07.05.2020 10:58

English, 07.05.2020 10:58


Social Studies, 07.05.2020 10:58

English, 07.05.2020 10:58

Computers and Technology, 07.05.2020 10:58

Mathematics, 07.05.2020 10:58

Mathematics, 07.05.2020 10:58

Mathematics, 07.05.2020 10:58

Mathematics, 07.05.2020 10:58



Mathematics, 07.05.2020 10:58

Mathematics, 07.05.2020 10:58


Mathematics, 07.05.2020 10:58


English, 07.05.2020 10:58

Spanish, 07.05.2020 10:58

English, 07.05.2020 10:58