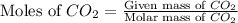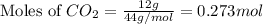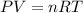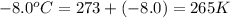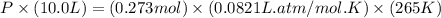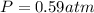Chemistry, 23.04.2020 01:05 MrTeriffic
A reaction between liquid reactants takes place at -8.0 C in a sealed, evacuated vessel with a measured volume of 10.0 L. Measurements show that the reaction produced 12 g or carbon dioxide gas. Calculate the pressure of carbon dioxide in the reaction vessel after the reaction. You may ignore the volume of the liquid reactants. Round you answer to 2 significant digits.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:30
Select the correct text in the passage. which sentences describe examples of sustainable living? i live in an old apartment building downtown, but my company is based in an office park on the outskirts of the city. i drive an old car that needs to be replaced. i plan to buy a hybrid for better gas mileage, but for now i am able to carpool with a couple of friends from work. the drive to the office park is about 45 minutes each way, but we do get to work in a modern building. the architects just received a leed certification for the design.
Answers: 3

Chemistry, 22.06.2019 19:00
Which change to the system wood cause the freely-moving piston to lower?
Answers: 1

Chemistry, 22.06.2019 21:50
28. which is not a reason that water is used to store spent fuel rods from nuclear power plants? water increases the speed of the chain reaction in the fuel rods. water protects nuclear power plant workers from the high temperature and radiation of the fuel rods. water acts as a radiation shield to reduce the radiation levels. water cools the spent rods. salts action
Answers: 1

Chemistry, 23.06.2019 14:00
How many moles of oxygens atoms are present in 5.00 mol of diphosphorus of fe2(so4)3
Answers: 2
You know the right answer?
A reaction between liquid reactants takes place at -8.0 C in a sealed, evacuated vessel with a measu...
Questions


Mathematics, 14.05.2021 18:00





Mathematics, 14.05.2021 18:00

Biology, 14.05.2021 18:00



Geography, 14.05.2021 18:00

Physics, 14.05.2021 18:00

Arts, 14.05.2021 18:00

Social Studies, 14.05.2021 18:00


Health, 14.05.2021 18:00

Mathematics, 14.05.2021 18:00

Mathematics, 14.05.2021 18:00

Mathematics, 14.05.2021 18:00

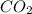 = 12 g
= 12 g