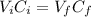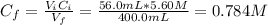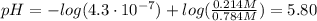Chemistry, 22.04.2020 22:28 ayowazzzgood
To prepare a buffer you weigh out 7.20 grams of NaHCO3 and place it into a 400.00 mL volumetric flask. To this flask you add 56.0 mL of 5.60 M H2CO3 and then fill it about halfway with distilled water, swirling to dissolve the contents. Finally, the flask is filled the rest of the way to the mark with distilled water. What is the pH of the buffer that you have created?Acid KaH2CO3 4.3 X 10⁻⁷ HCN 4.9 X 10⁻¹⁰HNO2 4.6 X 10⁻⁴C6H5COOH 6.5 X 10⁻⁵

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:00
This image is an example of a(n) a) atom. b) compound. c) mixture. d) molecule.
Answers: 1

Chemistry, 22.06.2019 19:00
Structure of the atoms: discovery of the nucleus in 1909i need answering all of these questions
Answers: 3

Chemistry, 23.06.2019 00:10
Covalent compounds: mastery test select the correct answer what is formed when atoms join together with a covalent bond? a. an ion b. a molecule c. a neutral atom d. a noble gas
Answers: 3

Chemistry, 23.06.2019 02:30
What role does weathering have in shaping earth’s surface? a) it allows sediments to fall out of a medium. b) it sediments settle on a new surface. c) it breaks down older material into sediments. d) it transports sediments to a different location. will give brainliest, answer quickly.
Answers: 2
You know the right answer?
To prepare a buffer you weigh out 7.20 grams of NaHCO3 and place it into a 400.00 mL volumetric flas...
Questions




Law, 14.07.2020 01:01


Mathematics, 14.07.2020 01:01

Mathematics, 14.07.2020 01:01



Social Studies, 14.07.2020 01:01

English, 14.07.2020 01:01



English, 14.07.2020 01:01

Chemistry, 14.07.2020 01:01

English, 14.07.2020 01:01

Mathematics, 14.07.2020 01:01

Mathematics, 14.07.2020 01:01

Mathematics, 14.07.2020 01:01

![pH = pKa + log(\frac{[NaHCO_{3}]}{[H_{2}CO_{3}]})](/tpl/images/0619/4920/b4240.png) (1)
(1)![[NaHCO_{3}] = \frac{mol}{V} = \frac{m}{M*V}](/tpl/images/0619/4920/e7247.png)
![[NaHCO_{3}] = \frac{7.20 g}{84.007 g/mol*400.0 \cdot 10^{-3} L} = 0.214 M](/tpl/images/0619/4920/c7722.png)