
Chemistry, 22.04.2020 20:00 taralynnn8870
When a 0.031M aqueous solution of a certain acid is prepared, the acid is 0.89% dissociated. Calculate the acid dissociation constant Ka of the acid. Round your answer to 2 significant digits.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:00
You mix the pks of succinic acid are 4.21 and 5.64. how many gramsa graduate student at sdsu wants to measure the activity of a particular enzyme at ph 4.0. to buffer her reaction, she will use a buffer system based on one of the acids listed below, which acid is most appropriate for the experiment? of monosodium succinate (fw = 140 g/mol) and disodium succinate (fw = 162 g/mol) must be added to 1 l of water to produce a solution with a ph 5.28 and a total solute concentration of 100 mm? (assume the total volume remains 1 liter, answer in grams monosodium succinate, grams disodium succinate, respectively.) volumes of 0.05 m nah2po4 and 0.05 m na2hpo4 (pk's for phosphoric acid are 2.15, 6.82 and 12.38). which of the following best describes the resulting solution?
Answers: 2

Chemistry, 22.06.2019 17:40
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1

Chemistry, 23.06.2019 01:30
Use the periodic table to determine how many grams of oxygen would be required to react completely with 859.0 g c2h2
Answers: 3

Chemistry, 23.06.2019 08:00
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1
You know the right answer?
When a 0.031M aqueous solution of a certain acid is prepared, the acid is 0.89% dissociated. Calcula...
Questions



English, 02.08.2021 20:50


Mathematics, 02.08.2021 20:50



Mathematics, 02.08.2021 20:50




Computers and Technology, 02.08.2021 20:50




Mathematics, 02.08.2021 20:50

Mathematics, 02.08.2021 20:50



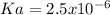
 due to the reaction extent, the percent dissociation is:
due to the reaction extent, the percent dissociation is:![\% Dissociation:\frac{x}{[acid]_0}](/tpl/images/0618/9112/63f66.png)
![x=\% Dissociation*[acid]_0=0.89\%*0.031M=2.759x10^{-4}M](/tpl/images/0618/9112/fb10b.png)
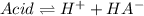
![Ka=\frac{x*x}{[acid]_0-x}=\frac{2.759x10^{-4}M*2.759x10^{-4}M}{0.031M-2.759x10^{-4}M} \\\\Ka=2.5x10^{-6}](/tpl/images/0618/9112/d4830.png)


