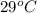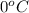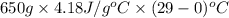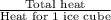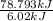Approximately how many ice cubes must melt to cool 650 milliliters of water from 29°C to 0°C? Assume that each ice cube contains 1 mole of H2O and is initially at 0°C. ∆H(fusion) = 6.02 kJ/mol; ∆H(vaporization) = 40.7 kJ/mol c(solid) = 2.09 J/g°C; c(liquid) = 4.18 J/g°C; c(gas) = 1.97 J/g°C Enter your answer numerically.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:30
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1

Chemistry, 22.06.2019 19:30
Awoman's basketball has a circumference between 28.5 and 29.0 inches and a maximum weight of 20 ounces (two significant figures). what are these specifications in units of centimeters and grams?
Answers: 2

Chemistry, 22.06.2019 22:00
How many moles of no2 will form when 3.3 moles of cu are reacted with excess hno3?
Answers: 3
You know the right answer?
Approximately how many ice cubes must melt to cool 650 milliliters of water from 29°C to 0°C? Assume...
Questions

Mathematics, 02.06.2021 23:50


Mathematics, 02.06.2021 23:50

Mathematics, 02.06.2021 23:50


Chemistry, 02.06.2021 23:50

Physics, 02.06.2021 23:50

Mathematics, 02.06.2021 23:50


History, 02.06.2021 23:50



Mathematics, 02.06.2021 23:50


Mathematics, 02.06.2021 23:50





Mathematics, 02.06.2021 23:50

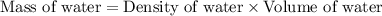
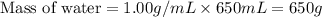
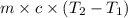
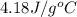
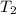 = final temperature =
= final temperature =