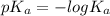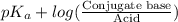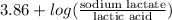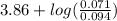"" What is the pH of a solution that is prepared by dissolving 8.52 grams of lactic acid (formula weight = 90.08 grams/mol) and 7.93 grams of sodium lactate (formula weight = 112.06 grams/mole) in water and diluting to 500.00 mL? The Ka for lactic acid is 0.000137.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:30
Given that 1 mi = 1760 yd, determine what conver- sion factor is appropriate to convert 1849 yd to miles; to convert 2.781 mi to yards.
Answers: 2

Chemistry, 22.06.2019 10:40
If an area has high air pressure and low humidity, what type of weather will it most likely have? plz !
Answers: 1

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 22:40
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution.part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1
You know the right answer?
"" What is the pH of a solution that is prepared by dissolving 8.52 grams of lactic acid (formula we...
Questions



Chemistry, 10.12.2020 17:50

German, 10.12.2020 17:50



Spanish, 10.12.2020 17:50


Mathematics, 10.12.2020 17:50

History, 10.12.2020 17:50



Mathematics, 10.12.2020 17:50


History, 10.12.2020 17:50



Mathematics, 10.12.2020 17:50

Physics, 10.12.2020 17:50

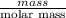
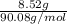
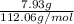
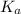 and
and 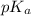 is as follows.
is as follows.