Chemistry, 21.04.2020 22:04 Irenesmarie8493
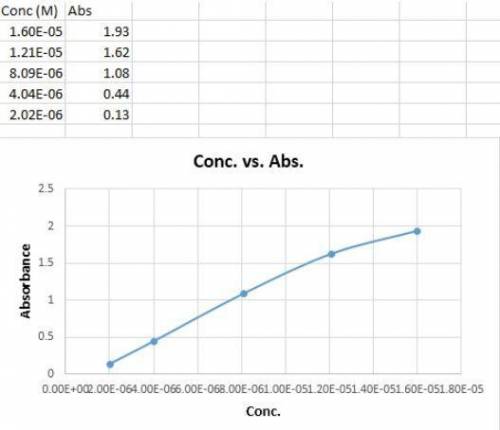
You make dilutions of curcumin stock solution and measure the absorbance of each dilution to obtain the following data: Conc. (M) Abs. 1.60E-05 1.93 1.21E-05 1.62 8.09E-06 1.08 4.04E-06 0.44 2.02E-06 0.13 Which data points should you keep when making your calibration curve? Choose all that apply. (Hint: Plot the data.)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:00
According to the tide table below what time of day will the highest tide occur?
Answers: 1

Chemistry, 22.06.2019 04:20
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 1

Chemistry, 23.06.2019 01:00
Imagine if during the cathode ray experiment, the size of the particles of the ray was the same as the size of the atom forming the cathode. which other model or scientific observation would have also been supported? 1. this would support dalton's postulates that proposed the atoms are indivisible because no small particles are involved. 2. this would support bohr's prediction about electrons moving in orbits having specific energy. 3. this would support bohr's prediction about electrons being randomly scattered around the nucleus in the atom. 4. this would support dalton's postulates that proposed that atoms combine in fixed whole number ratios to form compounds.
Answers: 1
You know the right answer?
You make dilutions of curcumin stock solution and measure the absorbance of each dilution to obtain...
Questions




Mathematics, 20.08.2020 21:01



Mathematics, 20.08.2020 21:01





Mathematics, 20.08.2020 21:01






History, 20.08.2020 21:01


History, 20.08.2020 21:01