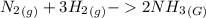Chemistry, 21.04.2020 20:17 amypeace1978
A sample of gas contains 0.1100 mol of N2(g) and 0.3300 mol of H2(g) and occupies a volume of 20.5 L. The following reaction takes place: N2(g) 3 H2(g) 2 NH3(g) Calculate the volume of the sample after the reaction takes place, assuming that the temperature and the pressure remain constant. g

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:50
Many reactions take place in aqueous solution. when potential reactants are mixed, a reaction will occur if there is some driving force that favors the formation of products. it is often convenient to categorize reactions in terms of these driving forces: precipitate formation, in which an insoluble solid is formed, weak electrolyte formation, as in a neutralization reaction involving water, or transfer of electrons, as in a redox reaction. these reactions can be represented by full molecular equations, which contain all species in the reaction mixture, or by net ionic equations, which show only the species that actually undergo a change. the latter does not contain the spectator ions, which do not undergo a net change or do not take part in the reaction. part a when the following two solutions are mixed: k2co3(aq)+fe(no3)3(aq) the mixture contains the ions listed below. sort these species into spectator ions and ions that react. drag the appropriate items to their respective bins. view available hint(s) spectator ions ions that react part b what is the correct net ionic equation, including all coefficients, charges, and phases, for the following set of reactants? assume that the contribution of protons from h2so4 is near 100 %.ba(oh)2(aq)+h2so4(aq)→ express your answer as a chemical equation. view available hint(s) nothing provide feedback
Answers: 3

Chemistry, 23.06.2019 04:31
Areaction is first order. if the initial reactant concentration is 0.0200 m, and 25.0 days later the concentration is 6.25 x 10-4 m, then its half-life is:
Answers: 1

Chemistry, 23.06.2019 04:40
[01.07]what is the answer to the problem: 101 g + 25.01 g + 5.05 g? 131.06 g 131.1 g 131 g 130 g
Answers: 1

Chemistry, 23.06.2019 08:00
Match the vocabulary terms to their definitions. 1 . a long, chain-like set of molecules made up of repeating units joined end to end polymer 2 . a hard, brittle, heat- and corrosion-resistant material made by subjecting a nonmetallic mineral mixture to intense heat ceramic 3 . a plastic with low elongations that cannot be recycled thermoset 4 . a carbon fiber embedded in a polymer resin matrix thermoplastic 5 . a plastic with high elongations that can be recycled crystal 6 . a solid form resulting from the arrangement of atoms, ions, or molecules in definite geometric patterns composite
Answers: 1
You know the right answer?
A sample of gas contains 0.1100 mol of N2(g) and 0.3300 mol of H2(g) and occupies a volume of 20.5 L...
Questions

Mathematics, 14.10.2019 22:00


Mathematics, 14.10.2019 22:00

History, 14.10.2019 22:00

Mathematics, 14.10.2019 22:00



Mathematics, 14.10.2019 22:00

English, 14.10.2019 22:00


Business, 14.10.2019 22:00

Chemistry, 14.10.2019 22:00

English, 14.10.2019 22:00


History, 14.10.2019 22:00


Mathematics, 14.10.2019 22:00


Physics, 14.10.2019 22:00

Business, 14.10.2019 22:00