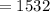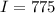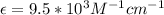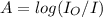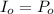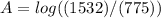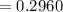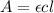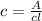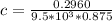Chemistry, 16.04.2020 01:01 Scienceissofun6453
Calculate the concentration of an anthracene solution which produced a fluorescence intensity ( I ) of 775 when the irradiance of the beam incident to the sample ( P 0 ) was 1532 and the length of the medium ( b ) was 0.875 cm. Anthracene has a molar extinction coefficient ( ϵ ) of 9.5 × 10 3 M − 1 ⋅ cm − 1 . The proportionality constant k ′ for anthracene is 0.30.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 17:00
In the analysis of hair and fiber samples, which does a compound comparison microscope allow for that a conventional compound microscope does not? a. simultaneous observation b. polarization c. fluorescence d. higher magnification
Answers: 2



Chemistry, 22.06.2019 05:10
How many miles of water are produced if 5.43 mol pbo2 are consumed
Answers: 1
You know the right answer?
Calculate the concentration of an anthracene solution which produced a fluorescence intensity ( I )...
Questions




Mathematics, 13.01.2021 18:10


English, 13.01.2021 18:10

Chemistry, 13.01.2021 18:10


Mathematics, 13.01.2021 18:10

Chemistry, 13.01.2021 18:10



Chemistry, 13.01.2021 18:10







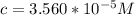
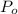 is
is