
The following reaction was used to fuel the rockets in the Apollo mission landing module.
A) Is this reaction endothermic or exothermic?
B) How many grams of N2H4 must be reacted with an excess of N2O4 to produce 775 kJ of energy?
C) How many kJ of energy are produced when 6.25 g of N2O4 reacts with an excess of N2H4?
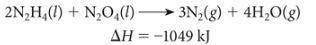

Answers: 2
Another question on Chemistry



Chemistry, 22.06.2019 06:30
Design techniques and materials that reduce the negative environmental impact of a structure are referred to as
Answers: 2

Chemistry, 22.06.2019 16:00
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1
You know the right answer?
The following reaction was used to fuel the rockets in the Apollo mission landing module.
Questions

Mathematics, 23.02.2021 02:30


Mathematics, 23.02.2021 02:30

Mathematics, 23.02.2021 02:30

Mathematics, 23.02.2021 02:30

Mathematics, 23.02.2021 02:30

Biology, 23.02.2021 02:30





Mathematics, 23.02.2021 02:30


Mathematics, 23.02.2021 02:30

Mathematics, 23.02.2021 02:30


History, 23.02.2021 02:30



Biology, 23.02.2021 02:30



