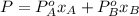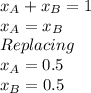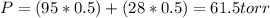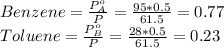Chemistry, 15.04.2020 20:08 lilakatedancer
Benzene and toluene form ideal solutions. Consider a solution of benzene and toluene prepared at 25 ^ { \circ } \mathrm { C }25∘C. Assuming that the mole fractions of benzene and toluene in the vapor phase are equal, calculate the composition of the solution. At 25 ^ { \circ } \mathrm { C }25∘C the vapor pressures of benzene and toluene are 95 and 28 torr, respectively.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 01:30
What is the value of keq for the reaction expressed in scientific notation
Answers: 1

Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2

Chemistry, 22.06.2019 22:50
At the current rate, a graph of carbon dioxide produced by fossil fuels over time would slope upward slope downward be horizontal be vertical
Answers: 3

Chemistry, 23.06.2019 04:20
Which activity describes an application of topographic maps? check all that apply. recreation, such as camping and hiking engineering, such as the construction of roads and buildings science, such as mapping stars in the sky business, such as analyzing population centers science, such as analyzing surface features
Answers: 1
You know the right answer?
Benzene and toluene form ideal solutions. Consider a solution of benzene and toluene prepared at 25...
Questions


History, 04.06.2020 06:58

History, 04.06.2020 06:58

Mathematics, 04.06.2020 06:58

Mathematics, 04.06.2020 06:58

History, 04.06.2020 06:58




Mathematics, 04.06.2020 06:58

Geography, 04.06.2020 06:58

Mathematics, 04.06.2020 06:58

History, 04.06.2020 06:58



Mathematics, 04.06.2020 06:58

English, 04.06.2020 06:58

History, 04.06.2020 06:58


English, 04.06.2020 06:59