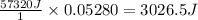A student enters the lab and conducts Part A of the Experiment. The student uses 25.00 mL of 2.112 M HCl, and adds NaOH in excess as instructed. If the ΔH of the neutralization reaction is known to be -57,320 J/mol H2O, what is the total theoretical heat released (in Joules)?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:00
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2

Chemistry, 22.06.2019 10:30
How do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 2

Chemistry, 22.06.2019 16:00
How do dying stars contribute to the formation of planets
Answers: 1

Chemistry, 22.06.2019 16:00
How could a student test the effect of removing heat from a gas that is stored in a sealed container? what must occur in order for matter to change states?
Answers: 2
You know the right answer?
A student enters the lab and conducts Part A of the Experiment. The student uses 25.00 mL of 2.112 M...
Questions

History, 08.04.2021 01:00

History, 08.04.2021 01:00

Spanish, 08.04.2021 01:00

Mathematics, 08.04.2021 01:00

Mathematics, 08.04.2021 01:00

Biology, 08.04.2021 01:00

Mathematics, 08.04.2021 01:00


Mathematics, 08.04.2021 01:00




Mathematics, 08.04.2021 01:00

History, 08.04.2021 01:00

History, 08.04.2021 01:00



English, 08.04.2021 01:00

Mathematics, 08.04.2021 01:00

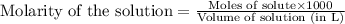 .....(1)
.....(1)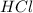 solution = 2.112 M
solution = 2.112 M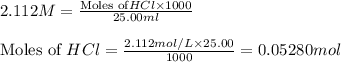

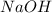 is the excess reagent.
is the excess reagent.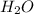
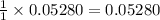 moles of
moles of