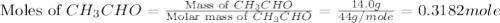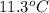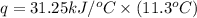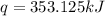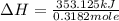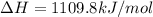Chemistry, 15.04.2020 03:33 tusharchandler124
The heat capacity of a bomb calorimeter was determined by burning 6.91 g of methane (energy of combustion = −803 kJ/mol CH4) in the bomb. The temperature changed by 11.1°C. (a) What is the heat capacity of the bomb? kJ/°C (b) A 14.0-g sample of acetaldehyde (CH3CHO) produced a temperature increase of 11.3°C in the same calorimeter. What is the energy of combustion of acetaldehyde (in kJ/mol)?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which statement best describes the oxidation numbers of the atoms found in magnesium chloride? a. magnesium has a 2- oxidation number and chlorine has a 1+ oxidation number. b. magnesium has a 2- oxidation number and chlorine has a 2+ oxidation number. c. magnesium has a 2+ oxidation number and chlorine has a 1- oxidation number. d. magnesium has a 1+ oxidation number and chlorine has a 1- oxidation number.
Answers: 2


Chemistry, 22.06.2019 16:00
Which factor is likely to impact the possible number of compounds ?
Answers: 1

Chemistry, 22.06.2019 17:10
Increasing the substrate concentration in an enzymatic reaction could overcome which of the following? a) the need for a coenzymeb) allosteric inhibitionc) competitive inhibitiond) insufficient cofactors
Answers: 1
You know the right answer?
The heat capacity of a bomb calorimeter was determined by burning 6.91 g of methane (energy of combu...
Questions


History, 11.01.2020 22:31

Physics, 11.01.2020 22:31



English, 11.01.2020 22:31

History, 11.01.2020 22:31






History, 11.01.2020 22:31

Biology, 11.01.2020 22:31






History, 11.01.2020 22:31

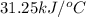
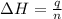
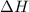 = enthalpy change = -803 kJ/mol
= enthalpy change = -803 kJ/mol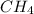 = 6.91 g
= 6.91 g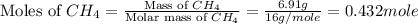
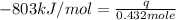
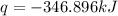
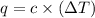
 = change in temperature =
= change in temperature = 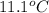
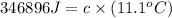
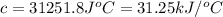
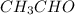 = 14.0 g
= 14.0 g