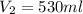Chemistry, 15.04.2020 03:01 Amazingboliver4518
A sample of nitrogen gas had a volume of 500. mL, a pressure in its closed container of 740 torr, and a temperature of 25 °C. What was the new volume of the gas when the temperature was changed to 50 °C and the new pressure was 760 torr, if the amount of gas does not change?
A) 530 mL
B) 450 ml
C) 970 mL
D) 240 ml
E) 400 mL

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:30
According to periodic trend, which of the following most likely has the highest ionization energy? kr be ni sc
Answers: 3

Chemistry, 22.06.2019 10:00
Why is the structure of molecule important to its function?
Answers: 1

Chemistry, 22.06.2019 12:00
There is one girl i like and i don't know how to tell her that, i have a feeling she knows but if she doesn't i don't want to make a fool out of myself how is one way to boost my confidence on asking her out
Answers: 1

Chemistry, 22.06.2019 20:00
Phosphoric acid is a triprotic acid ( =6.9×10−3 , =6.2×10−8 , and =4.8×10−13 ). to find the ph of a buffer composed of h2po−4(aq) and hpo2−4(aq) , which p value should be used in the henderson–hasselbalch equation? p k a1 = 2.16 p k a2 = 7.21 p k a3 = 12.32 calculate the ph of a buffer solution obtained by dissolving 18.0 18.0 g of kh2po4(s) kh 2 po 4 ( s ) and 33.0 33.0 g of na2hpo4(s) na 2 hpo 4 ( s ) in water and then diluting to 1.00 l.
Answers: 3
You know the right answer?
A sample of nitrogen gas had a volume of 500. mL, a pressure in its closed container of 740 torr, an...
Questions



Arts, 15.01.2021 18:40

Mathematics, 15.01.2021 18:40



Mathematics, 15.01.2021 18:40

Arts, 15.01.2021 18:40


Mathematics, 15.01.2021 18:40



English, 15.01.2021 18:40

Mathematics, 15.01.2021 18:40


Mathematics, 15.01.2021 18:40

Mathematics, 15.01.2021 18:40


Mathematics, 15.01.2021 18:40

Mathematics, 15.01.2021 18:40


 = initial pressure of gas = 740 torr
= initial pressure of gas = 740 torr = final pressure of gas = 760 torr
= final pressure of gas = 760 torr = initial volume of gas = 500 ml
= initial volume of gas = 500 ml = final volume of gas = ?
= final volume of gas = ? = initial temperature of gas =
= initial temperature of gas = 
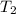 = final temperature of gas =
= final temperature of gas =