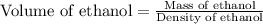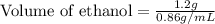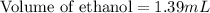Chemistry, 15.04.2020 02:56 Blazingangelkl
Recrystallization Step: The procedure asks for using equal amount by mass of the crude product and the recrystallization solvent, 95% ethanol. Suppose you use 1.2g of product, how many mL of 95% ethanol would you add? Write the answer as a number (without units) with 3 significant figures (e. g. 2.15). Density of 95% ethanol = 0.86 g/mL

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:00
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1

Chemistry, 22.06.2019 14:00
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2

Chemistry, 22.06.2019 20:00
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. in the first step, calcium carbide and water react to form acetylene and calcium hydroxide: cac2 (s) + 2h2o (g) → c2h2 (g) + caoh2 (s) =δh−414.kj in the second step, acetylene, carbon dioxide and water react to form acrylic acid: 6c2h2 (g) + 3co2 (g) + 4h2o (g) → 5ch2chco2h (g) =δh132.kj calculate the net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide from these reactions. round your answer to the nearest kj .
Answers: 3

Chemistry, 23.06.2019 02:30
Apound is approximately 0.45 kilogram. a persons weighs 87 kilograms. what is the persons’s weight, in pounds, when expressed to the correct number of significant figures
Answers: 1
You know the right answer?
Recrystallization Step: The procedure asks for using equal amount by mass of the crude product and t...
Questions


Mathematics, 13.10.2019 13:10


Mathematics, 13.10.2019 13:10

History, 13.10.2019 13:10

Mathematics, 13.10.2019 13:10


English, 13.10.2019 13:10

Mathematics, 13.10.2019 13:10


Biology, 13.10.2019 13:10

Arts, 13.10.2019 13:10


Mathematics, 13.10.2019 13:10



History, 13.10.2019 13:10

Mathematics, 13.10.2019 13:10

Mathematics, 13.10.2019 13:10