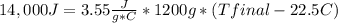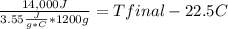Chemistry, 14.04.2020 22:50 emmaraeschool
Complete combustion of a 0.350 g sample of a compound in a bomb calorimeter releases 14.0 kJ of heat. The bomb calorimeter has a mass of 1.20 kg and a specific heat of 3.55 J/(gi°C). If the initial temperature of the calorimeter is 22.5°C, what is its final temperature? Use q equals m C subscript p Delta T..

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:30
For each of the following mixtures decide if filtering would be suitable to separate the substances. explain your answers. oil in water sugar in water sand in water chalk in water tea leaves in a cup of tea
Answers: 2


Chemistry, 21.06.2019 22:30
Which traits do human embryos have that link them to a common ancestor with fish and reptiles? a. scales and tail b. gill slits and scales c. tail and gill slits d. hair and tail
Answers: 2

Chemistry, 22.06.2019 01:40
C3h8o3 - glycerol major species present when dissolved in water
Answers: 2
You know the right answer?
Complete combustion of a 0.350 g sample of a compound in a bomb calorimeter releases 14.0 kJ of heat...
Questions



History, 29.01.2020 12:42


English, 29.01.2020 12:42


Mathematics, 29.01.2020 12:42

Computers and Technology, 29.01.2020 12:42



Mathematics, 29.01.2020 12:42



English, 29.01.2020 12:42



Mathematics, 29.01.2020 12:42



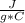 m=1.20 kg= 1200 g (1 kg=1000 g)Tfinal= ?Tinitial= 22.5 °C
m=1.20 kg= 1200 g (1 kg=1000 g)Tfinal= ?Tinitial= 22.5 °C