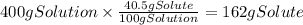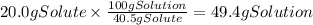Chemistry, 14.04.2020 22:30 hannahblank2466
A chemistry student needs of acetic acid for an experiment. He has available of a w/w solution of acetic acid in acetone. Calculate the mass of solution the student should use. If there's not enough solution, press the "No solution" button. Round your answer to significant digits.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
How much heat is released upon converting one mole of steam (18.0 g) from 100.0 ∘c to water at 25.0 ∘c? show work and constants, trying to figure out how it works. only given the heat capacity for steam and water so try to only use that
Answers: 1

Chemistry, 22.06.2019 09:00
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1

Chemistry, 22.06.2019 10:00
Main expenses you plan on making payments on a new car too. you want to spend 15% of your monthly net pay on the car payment, insurance, registration, and taxes combined. what is your monthly car allowance? $149.46 $298.91 $448.37 $597.83
Answers: 3

Chemistry, 22.06.2019 12:30
What metric units would you use to measure the thickness of a key
Answers: 3
You know the right answer?
A chemistry student needs of acetic acid for an experiment. He has available of a w/w solution of ac...
Questions

History, 13.06.2020 21:57














Mathematics, 13.06.2020 21:57



Mathematics, 13.06.2020 21:57

Mathematics, 13.06.2020 21:57

History, 13.06.2020 21:57