
Chemistry, 14.04.2020 22:20 casting479
A sample of hydrogen gas occupies a volume of 6.38 L at 48.0°C and 0.490 atm. If it is desired to increase the volume of the gas sample to 8.10 L, while increasing its pressure to 0.655 atm, the temperature of the gas sample at the new volume and pressure must be °C.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:00
When determining the shape of a molecule, it is important to draw a lewis dot structure first in order to see the total number a. electrons within the moleculeb. bonding and unshared pairs around central atomc. unshared pair within the molecule( i really need it )
Answers: 1

Chemistry, 22.06.2019 08:30
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2

Chemistry, 22.06.2019 10:50
How many liters of oxygen gas, at standard temperature and pressure, will react with 35.8 grams of iron metal? 4 fe (s) + 3 o₂ (g) → 2 fe₂o₃ (s)
Answers: 2

Chemistry, 22.06.2019 12:00
the mississippians were considered to be horticulturalists, which means they were
Answers: 1
You know the right answer?
A sample of hydrogen gas occupies a volume of 6.38 L at 48.0°C and 0.490 atm. If it is desired to in...
Questions


Business, 30.09.2019 07:30

Mathematics, 30.09.2019 07:30

Physics, 30.09.2019 07:30


Mathematics, 30.09.2019 07:30

Biology, 30.09.2019 07:30

Mathematics, 30.09.2019 07:30


Health, 30.09.2019 07:30

Business, 30.09.2019 07:30


Chemistry, 30.09.2019 07:30


Biology, 30.09.2019 07:30


Mathematics, 30.09.2019 07:30



Mathematics, 30.09.2019 07:30

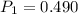 atm
atm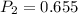 atm
atm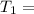 48° C = 321 K
48° C = 321 K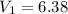 L
L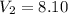 L
L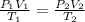
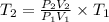
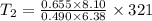
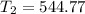 K
K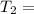 271.77°C
271.77°C

