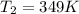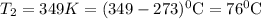Mount Everest rises to a height of 8,850 m above sea level. At a base camp on the mountain the atmospheric pressure is measured to be 314.0 mm Hg. At what temperature (in °C) will water boil at base camp ? The vapor pressure of water at 373 K is 760.0 mm Hg. (ΔH°vap for H2O = 40.7 kJ/mol and R = 8.314 J/mol K).
a. 344°C.
b. 70.8°C .
c. 2.91E-3°C .
d. 79.8°C.
e. 57.8°C.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:30
Based on the equation and the information in the table, what is the enthalpy of the reaction?
Answers: 2


Chemistry, 23.06.2019 02:40
How can a mixture of salt water be separated into salt and water
Answers: 1

Chemistry, 23.06.2019 05:40
Why is it incorrect to balance a chemical equation by changing the subscripts? explain.
Answers: 2
You know the right answer?
Mount Everest rises to a height of 8,850 m above sea level. At a base camp on the mountain the atmos...
Questions

Mathematics, 19.04.2020 01:18





Social Studies, 19.04.2020 01:18


Biology, 19.04.2020 01:18

Biology, 19.04.2020 01:18






Social Studies, 19.04.2020 01:18


Law, 19.04.2020 01:18



Mathematics, 19.04.2020 01:18

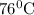 .
.![ln(\frac{P_{2}}{P_{1}})=\frac{-\Delta H_{vap}^{0}}{R}[\frac{1}{T_{2}}-\frac{1}{T_{1}}]](/tpl/images/0599/0769/06906.png)
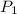 and
and 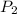 are vapor pressures of liquid at
are vapor pressures of liquid at 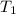 (in kelvin) and
(in kelvin) and 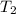 (in kelvin) temperatures respectively.
(in kelvin) temperatures respectively.![ln(\frac{314.0}{760.0})=\frac{-40.7\times 10^{3}\frac{J}{mol}}{8.314\frac{J}{mol.K}}\times [\frac{1}{T_{2}}-\frac{1}{373K}]](/tpl/images/0599/0769/811c1.png)