Chemistry, 14.04.2020 17:01 ashiteru123
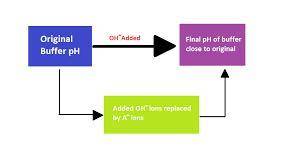
A 1.50 L buffer solution consists of 0.118 M butanoic acid and 0.318 M sodium butanoate. Calculate the pH of the solution following the addition of 0.063 moles of NaOH . Assume that any contribution of the NaOH to the volume of the solution is negligible. The K a of butanoic acid is 1.52 × 10 − 5 .

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:00
The image shows a process that releases nuclear energy which statement best identifies the process shown the process must be fusion because energy is released the process must be fusion because of have your nucleus formed a smaller nuclei the process must be fission because a large nucleus breaks into smaller nuclei the process must be fission because neutrons are formed
Answers: 1

Chemistry, 22.06.2019 06:00
Calculate the mass of silver needed to react with chlorine to produce 126g if silver chloride?
Answers: 3

Chemistry, 22.06.2019 10:30
Geothermal energy for industrial use is available almost anywhere. a.true b.false
Answers: 2

Chemistry, 22.06.2019 16:10
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1
You know the right answer?
A 1.50 L buffer solution consists of 0.118 M butanoic acid and 0.318 M sodium butanoate. Calculate t...
Questions


Mathematics, 30.06.2019 23:30

Mathematics, 30.06.2019 23:30

Mathematics, 30.06.2019 23:30

Mathematics, 30.06.2019 23:30

Geography, 30.06.2019 23:30






Mathematics, 30.06.2019 23:30

Mathematics, 30.06.2019 23:30

Mathematics, 30.06.2019 23:30





History, 30.06.2019 23:30

Physics, 30.06.2019 23:30