
Chemistry, 08.04.2020 20:07 Darkknighta26
The reaction 2 n2o5(g) → 4 no2(g) + o2(g) is found to obey the rate law: rate = k [n2o5]. does the reaction mechanism consist of only a single bimolecular step? 1. yes, it must 2. it might, but you cannot tell from the information given. 3. no, it cannot

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 08:00
If 90.0 grams of ethane reacted with excess chlorine,how many grams of dicarbon hexachloride would form
Answers: 1

Chemistry, 22.06.2019 11:30
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3

Chemistry, 22.06.2019 12:00
Which of the following is an example of physical change not a chemical change? a) a log gives off heat and light as it burns. b) a tree stores energy from the sun in its fruit. c) a penny lost in the grass slowly changes color. d) a water pipe freezes and cracks on a cold night.
Answers: 2

Chemistry, 22.06.2019 13:30
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1
You know the right answer?
The reaction 2 n2o5(g) → 4 no2(g) + o2(g) is found to obey the rate law: rate = k [n2o5]. does the r...
Questions


Mathematics, 23.10.2020 01:01

Computers and Technology, 23.10.2020 01:01



History, 23.10.2020 01:01

English, 23.10.2020 01:01


Mathematics, 23.10.2020 01:01


Mathematics, 23.10.2020 01:01


Mathematics, 23.10.2020 01:01


Computers and Technology, 23.10.2020 01:01

History, 23.10.2020 01:01


English, 23.10.2020 01:01


English, 23.10.2020 01:01

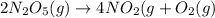
![Rate=k[N_2O_5]^1](/tpl/images/0589/8713/ae447.png)
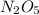 , it is a complex reaction.
, it is a complex reaction.

