Chemistry, 08.04.2020 19:24 pedroramirezr2
For many purposes we can treat nitrogen N2 as an ideal gas at temperatures above its boiling point of −196.°C. Suppose the temperature of a sample of nitrogen gas is lowered from 18.0°C to −15.0°C, and at the same time the pressure is changed. If the initial pressure was 3.6atm and the volume decreased by 40.0%, what is the final pressure? Round your answer to the correct number of significant digits.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which of the following statements is true about planck’s law
Answers: 1


Chemistry, 22.06.2019 09:00
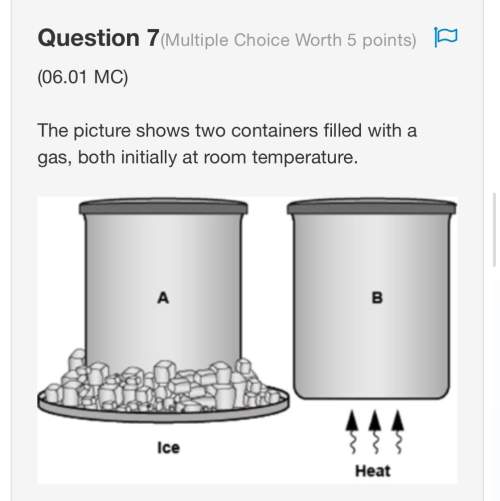
Which process does not require the presence of a physical substance in order to transfer heat? air in the atmosphere is heated by the ground. this warm air then rises, and cooler air falls. this is an example of what type of process? how is conduction different from radiation?
Answers: 1

Chemistry, 22.06.2019 09:40
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3
You know the right answer?
For many purposes we can treat nitrogen N2 as an ideal gas at temperatures above its boiling point o...
Questions


Business, 09.07.2019 21:00


Spanish, 09.07.2019 21:00



Social Studies, 09.07.2019 21:00


Biology, 09.07.2019 21:00






Computers and Technology, 09.07.2019 21:00

Biology, 09.07.2019 21:00




English, 09.07.2019 21:00



 is the initial temperature of the gas
is the initial temperature of the gas is the final temperature
is the final temperature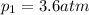 is the initial pressure
is the initial pressure , as the volume is decreased by 40.0%
, as the volume is decreased by 40.0% , we find the final pressure of the gas:
, we find the final pressure of the gas: