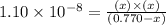Chemistry, 08.04.2020 19:24 jasmine2919
For the reaction below, Kc = 1.10 × 10⁻⁸. What is the equilibrium concentration of OH⁻ if the reaction begins with 0.770 M HONH₂?
HONH₂ (aq) + H₂O (l) ⇌ HONH₃⁺ (aq) + OH⁻ (aq)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:00
Drug abuse will not lead to physical and psychological dependence. true or false ?
Answers: 2

Chemistry, 22.06.2019 12:00
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1

Chemistry, 22.06.2019 13:30
Table sugar completely dissolved in water is an example of a?
Answers: 1

Chemistry, 22.06.2019 22:00
Choose all the answers that apply. fluorine (f) has an atomic number of 9 and an atomic weight of 18.99. fluorine has a. 9 protons b. 10 neutrons c. 18 electrons d. an atomic mass of 19 e. at least one isotope
Answers: 1
You know the right answer?
For the reaction below, Kc = 1.10 × 10⁻⁸. What is the equilibrium concentration of OH⁻ if the reacti...
Questions


English, 21.01.2021 23:40


Mathematics, 21.01.2021 23:40

Mathematics, 21.01.2021 23:40




Mathematics, 21.01.2021 23:40


Mathematics, 21.01.2021 23:40

Mathematics, 21.01.2021 23:40

Mathematics, 21.01.2021 23:40







Mathematics, 21.01.2021 23:40

 at equilibrium is 0.000092 M
at equilibrium is 0.000092 M

![K_c=\frac{[HONH_3^+]\times [OH^-]}{[HONH_2]}](/tpl/images/0589/8195/4bf94.png)