12.0 g NaHSO4 is dissolved in water to make a 2.00 L solution.
What is the molarity of the M...

Chemistry, 08.04.2020 17:20 jordantay208
12.0 g NaHSO4 is dissolved in water to make a 2.00 L solution.
What is the molarity of the M

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:30
Electrons are extremely important to what area of technology? a) anti-aging research b) household product development c) electronics d) drug discovery
Answers: 3

Chemistry, 22.06.2019 05:50
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

Chemistry, 22.06.2019 14:30
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1

Chemistry, 22.06.2019 16:00
Sulfuric acid is a polyprotic acid. write balanced chemical equations for the sequence of reactions that sulfuric acid can undergo when it's dissolved in water.
Answers: 2
You know the right answer?
Questions



Biology, 16.10.2020 14:01

Social Studies, 16.10.2020 14:01


Mathematics, 16.10.2020 14:01


Chemistry, 16.10.2020 14:01

Mathematics, 16.10.2020 14:01


Mathematics, 16.10.2020 14:01

History, 16.10.2020 14:01



Mathematics, 16.10.2020 14:01





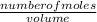
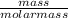
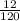 = 0.1mole
= 0.1mole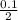 = 0.05M or 0.05moldm⁻³
= 0.05M or 0.05moldm⁻³

