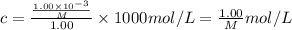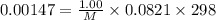A chemist is trying to determine the molar mass of a certain protein. 1.00 x 10 -3 g of it was dissolved in enough water to make 1.00 mL of solution. The osmotic pressure of this solution was found to be 1.12 torr at 25.0°C. Calculate the molar mass of the protein.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Each of the following compounds contains a metal that can exhibit more than one ionic charge. provide systematic names for each of these compounds. (a) cr(clo3)6 (b) mo(cn)6 (c) cr2(so3)3 (d) v(clo2)2 (e) v(cn)5 (f) os(clo2)4
Answers: 3

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1

Chemistry, 22.06.2019 23:30
To find the work done, the force exerted and distance moved are multiplied. a couch is moved twice before you are happy with its placement. the same force was used to move the couch both times. if more work is done the first time it is moved, what do you know about the distance it was moved? a) when more work was done, the couch was moved the same distance. b) when more work was done, the couch was moved less. c) when more work was done, the couch was moved further. d) when more work was done, the couch wasn't moved at all.
Answers: 1

You know the right answer?
A chemist is trying to determine the molar mass of a certain protein. 1.00 x 10 -3 g of it was disso...
Questions


English, 18.03.2021 03:30

Mathematics, 18.03.2021 03:30


Mathematics, 18.03.2021 03:30



Mathematics, 18.03.2021 03:30

Physics, 18.03.2021 03:30







Mathematics, 18.03.2021 03:30




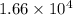 g/mol
g/mol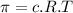
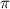 represents osmotic pressure of solution, c represents molarity of solution and T represents temperature in kelvin scale.
represents osmotic pressure of solution, c represents molarity of solution and T represents temperature in kelvin scale.