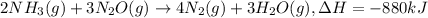When 2 moles of NH3(g) react with N2O(g) to form N2(g) and H2O(g) according to the following equation, 880 kJ of energy are evolved. 2NH3(g) + 3N2O(g)4N2(g) + 3H2O(g) Is this reaction endothermic or exothermic? What is the value of q? kJ An error has been detected in your answer. Che

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:20
Why does the sun appear to be the brightest star in the sky? a- its apparent brightness is much greater than other stars. b- it burns more gas, making it brighter than any other star. c- it is the largest star in the galaxy, so it is the brightest star. d- its relative distance to earth is closer than the other stars.
Answers: 1


Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1

Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2
You know the right answer?
When 2 moles of NH3(g) react with N2O(g) to form N2(g) and H2O(g) according to the following equatio...
Questions

Mathematics, 02.03.2021 18:20



Mathematics, 02.03.2021 18:20


Mathematics, 02.03.2021 18:20


Mathematics, 02.03.2021 18:20


Social Studies, 02.03.2021 18:20

Mathematics, 02.03.2021 18:20

English, 02.03.2021 18:20



Mathematics, 02.03.2021 18:20


Social Studies, 02.03.2021 18:20

English, 02.03.2021 18:20

Mathematics, 02.03.2021 18:20

Spanish, 02.03.2021 18:20

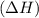 comes out to be negative.
comes out to be negative.