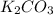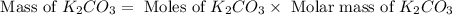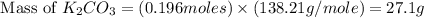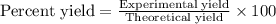Chemistry, 07.04.2020 22:58 timjape3g3z
Determine the theoretical yield and the percent yield if 21.8 g of KCO is produced from reacting 27.9 g KO with 29.0 L of CO (at STP). The molar mass of KO = 71.10 g/mol and KCO = 138.21 g/mol. 4 KO(s) + 2 CO(g) → 2 KCO(s) + 3 O(g)

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 19:30
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2

Chemistry, 22.06.2019 22:30
Which of the following is not an assumption that scientists must make about the natural world? a. regularity b. causality c. predictability d. plausibility
Answers: 1

Chemistry, 23.06.2019 04:00
Which of these are physical changes in matter? check all that apply boiling water a pencil being sharpened exploding dynamite freezing water rotting cheese
Answers: 1

Chemistry, 23.06.2019 04:40
[01.07]what is the answer to the problem: 101 g + 25.01 g + 5.05 g? 131.06 g 131.1 g 131 g 130 g
Answers: 1
You know the right answer?
Determine the theoretical yield and the percent yield if 21.8 g of KCO is produced from reacting 27....
Questions





Social Studies, 11.05.2021 21:40



Mathematics, 11.05.2021 21:40


Mathematics, 11.05.2021 21:40


History, 11.05.2021 21:40

Mathematics, 11.05.2021 21:40

English, 11.05.2021 21:40



Mathematics, 11.05.2021 21:40



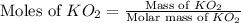
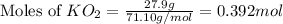
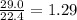 mole of CO₂ gas.
mole of CO₂ gas.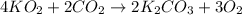
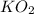 react with 2 mole of
react with 2 mole of 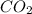
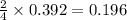 moles of
moles of