Chemistry, 07.04.2020 23:35 maxy7347go
How much energy must be removed from a 94.4 g sample of benzene (molar mass= 78.11 g/mol) at 322.0 K to solidify the sample and lower the temperature to 205.0 K? The following physical data may be useful. ΔHvap = 33.9 kJ/mol ΔHfus = 9.8 kJ/mol Cliq = 1.73 J/g°C Cgas = 1.06 J/g°C Csol = 1.51 J/g°C Tmelting = 279.0 K Tboiling = 353.0 K

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:00
What are the variables in gay-lussac’s law? pressure and volume pressure, temperature, and volume pressure and temperature volume, temperature, and moles of gas
Answers: 1

Chemistry, 22.06.2019 19:10
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1

Chemistry, 22.06.2019 23:00
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
Answers: 3

You know the right answer?
How much energy must be removed from a 94.4 g sample of benzene (molar mass= 78.11 g/mol) at 322.0 K...
Questions


History, 15.04.2021 18:50

History, 15.04.2021 18:50


Chemistry, 15.04.2021 18:50



Chemistry, 15.04.2021 18:50



Mathematics, 15.04.2021 18:50


Chemistry, 15.04.2021 18:50

Chemistry, 15.04.2021 18:50

Mathematics, 15.04.2021 18:50



Mathematics, 15.04.2021 18:50

Mathematics, 15.04.2021 18:50

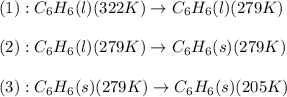
![Q=[m\times c_{p,l}\times (T_{final}-T_{initial})]+[m\times \Delta H_{fusion}]+[m\times c_{p,s}\times (T_{final}-T_{initial})]](/tpl/images/0587/9526/727a2.png)
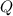 = heat released for the reaction = ?
= heat released for the reaction = ?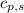 = specific heat of solid benzene =
= specific heat of solid benzene = 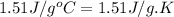
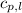 = specific heat of liquid benzene =
= specific heat of liquid benzene = 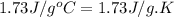
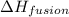 = enthalpy change for fusion =
= enthalpy change for fusion = 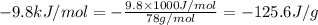
![Q=[94.4g\times 1.73J/g.K\times (279-322)K]+[94.4g\times -125.6J/g]+[94.4g\times 1.51J/g.K\times (205-279)K]](/tpl/images/0587/9526/0dc0d.png)