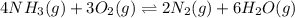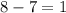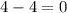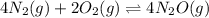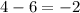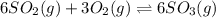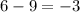Chemistry, 07.04.2020 17:24 19thomasar
In which of the following reactions will Kc= Kp?A) 4 NH3(g) + 3 O2(g) ⇌2 N2(g) + 6 H2O(g)B) 2 SO3(g) + 2 NO(g) ⇌2 SO2(g) + 2 NO2(g)C) 4 N2(g) + 2 O2(g) ⇌4 N2O(g)D) 6 SO2(g) + 3 O2(g) ⇌6 SO3(g)E) None of theabove reactions have Kc= Kp

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 15:30
219 grams of iron (iii) oxide reacts with excess carbon according to the reaction equation shown below. fe2o3 + c → fe + co2 after a scientist performs the chemical reaction they find the actual yield of iron to be 57.4 grams. calculate the percent yield of this chemical reaction.
Answers: 1

Chemistry, 22.06.2019 03:40
Astudent is given a sample of a blue copper sulfate hydrate. he weighs the sample in a dry covered porcelain crucible and got a mass of 23.875 g for the crucible, lid, and sample. the mass of the empty crucible and lid was found earlier to be 22.652 g. he then heats the crucible to expel the water of hydration, keeping the crucible at red heat for 10 minutes with the lid slightly ajar. on colling, he finds the mass of crucible, lid, and contents to be 23.403 g. the sample was changed in the process to very light clue anhydrous cuso4. if there are again 100.0 g of hydrate, how many grams of cuso4 are in it? how many moles of cuso4? (hint: molar mass of cuso4 = 159.6 g / mole. what per cent of the hydrate is cuso4? you may convert the mass of cuso4 to moles.)
Answers: 3

Chemistry, 22.06.2019 04:20
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 14:50
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3
You know the right answer?
In which of the following reactions will Kc= Kp?A) 4 NH3(g) + 3 O2(g) ⇌2 N2(g) + 6 H2O(g)B) 2 SO3(g)...
Questions


History, 11.04.2021 15:00


Mathematics, 11.04.2021 15:00

English, 11.04.2021 15:00

Arts, 11.04.2021 15:10

Health, 11.04.2021 15:10

Mathematics, 11.04.2021 15:10



Mathematics, 11.04.2021 15:10



Mathematics, 11.04.2021 15:10

Mathematics, 11.04.2021 15:10


Mathematics, 11.04.2021 15:10

Mathematics, 11.04.2021 15:10

Mathematics, 11.04.2021 15:10


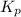 with
with 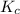 is given by the formula:
is given by the formula: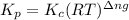
 = change in number of moles of gas particles =
= change in number of moles of gas particles = 
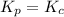 when
when