
Chemistry, 07.04.2020 17:03 ashley110608
Reaction A has a high activation energy, whereas reacton B has a low activation energy. Which of the statements about reaction A and reaction B are true? Reaction B is likely to occur at a faster rate than reaction A. Reaction A is more likely to occur at all than reaction B. Reaction B is more likely to occur at all than reaction A. Reaction A is likely to occur at a faster rate than reaction B.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
Which sentence best describes the formation of igneous rock? a- lava on the surface dries up and makes arock b_melted rocks cools and forms crystals c_rocks under tremendous heat and pressure d_magma is melted rock underground
Answers: 1


Chemistry, 22.06.2019 04:30
Suppose that during that icy hot lab 65,000 j of energy were transferred to 450 g of water at 20°c what would have have been the final temperature of the water
Answers: 2

You know the right answer?
Reaction A has a high activation energy, whereas reacton B has a low activation energy. Which of the...
Questions


Mathematics, 27.07.2019 13:40

Chemistry, 27.07.2019 13:40







Mathematics, 27.07.2019 13:40

Geography, 27.07.2019 13:40



Mathematics, 27.07.2019 13:40


Health, 27.07.2019 13:40




Chemistry, 27.07.2019 13:40

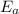 , and it is measured in Joules (J), KiloJoules (KJ) or Kilocalories per mole (Kcal/mol)
, and it is measured in Joules (J), KiloJoules (KJ) or Kilocalories per mole (Kcal/mol)

