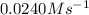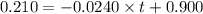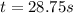Chemistry, 07.04.2020 15:59 angelricardoblp8kwg3
The rate constant for this zero‑order reaction is 0.0240 M ⋅ s − 1 0.0240 M·s−1 at 300 ∘ C. 300 ∘C. A ⟶ products A⟶products How long (in seconds) would it take for the concentration of A A to decrease from 0.900 M 0.900 M to 0.210 M?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 23.06.2019 06:30
Which of these natural resources is non-renewable a.corn b.wind c.geothermal d.natural gas
Answers: 2


Chemistry, 23.06.2019 18:30
What is the solution to the problem to the correct number of significant figures (102,900/12)+(170•1.27)
Answers: 1
You know the right answer?
The rate constant for this zero‑order reaction is 0.0240 M ⋅ s − 1 0.0240 M·s−1 at 300 ∘ C. 300 ∘C....
Questions

English, 15.04.2020 22:49












Mathematics, 15.04.2020 22:49



Biology, 15.04.2020 22:49

Business, 15.04.2020 22:49

Business, 15.04.2020 22:49

Business, 15.04.2020 22:49

![[A]=-kt+[A]_o](/tpl/images/0586/4094/d191d.png)
![[A]_o](/tpl/images/0586/4094/9caf5.png) = initial concentration = 0.900 M
= initial concentration = 0.900 M