
Chemistry, 04.04.2020 21:52 Shybaby5019
Sodium hydroxide is extremely soluble in water. At a certain temperature, a saturated solution contains 521 g of NaOH(s) per liter of solution. Calculate the molarity of this saturated NaOH(aq) solution.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 2

Chemistry, 22.06.2019 21:30
Isopropyl alcohol, (ch3)2choh, is a common solvent. determine the percent by mass of hydrogen in isopropyl alcohol. a) 6.71% h b) 13.4% h c) 25.0% h d) 53.3% h
Answers: 1

Chemistry, 23.06.2019 03:30
Select the correct lewis structure for fluorine which is group 7a element?
Answers: 1
You know the right answer?
Sodium hydroxide is extremely soluble in water. At a certain temperature, a saturated solution conta...
Questions




Mathematics, 16.02.2021 22:50

English, 16.02.2021 22:50

Mathematics, 16.02.2021 22:50



Mathematics, 16.02.2021 22:50

Chemistry, 16.02.2021 22:50




Mathematics, 16.02.2021 22:50


Spanish, 16.02.2021 22:50

History, 16.02.2021 22:50

Spanish, 16.02.2021 22:50


History, 16.02.2021 22:50

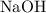 solution is approximately
solution is approximately 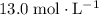 .
.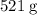 of
of 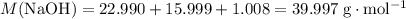 .
.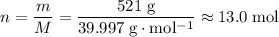 .
.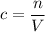 to find the molar concentration
to find the molar concentration  of this solution. In this equation,
of this solution. In this equation, 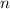 is the number of moles of the solute, and
is the number of moles of the solute, and 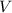 is the volume of the solution.
is the volume of the solution.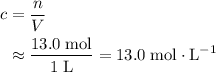 .
. 

