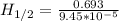Chemistry, 04.04.2020 14:06 munekalove69ounxwv
The isomerization of methylisonitrile to acetonitrile (CH3NC(g) CH3CN) is first order in CH3NC. The rate constant for the reaction is 9.45 x 10-5 s-1 at 478 K. What is the half-life of the reaction when the initial concentration of CH3NC is 0.0300 M?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 19:00
What information does a complete ionic equation give that the balanced equation doesn’t show?
Answers: 1

Chemistry, 22.06.2019 20:20
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3


Chemistry, 22.06.2019 22:30
Akno3 solution containing 51 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 3
You know the right answer?
The isomerization of methylisonitrile to acetonitrile (CH3NC(g) CH3CN) is first order in CH3NC. The...
Questions


Mathematics, 30.04.2021 14:00

Biology, 30.04.2021 14:00


Computers and Technology, 30.04.2021 14:00

History, 30.04.2021 14:00

Mathematics, 30.04.2021 14:00

Biology, 30.04.2021 14:00


Chemistry, 30.04.2021 14:00


Arts, 30.04.2021 14:00

Mathematics, 30.04.2021 14:00

Physics, 30.04.2021 14:00


Mathematics, 30.04.2021 14:00


Computers and Technology, 30.04.2021 14:00

Geography, 30.04.2021 14:00

World Languages, 30.04.2021 14:00

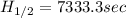
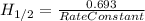
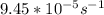 for the rate constant
for the rate constant