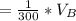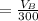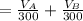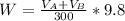Chemistry, 04.04.2020 14:09 vladsmolin7781
The solubility of aspirin in water is 1 g per 300 mL at 25 degrees celsius. Assuming that your crystallization and washing with water were done at this temperature, what weight of aspirin did you lose in the filtrate and washings? How much was your percent yield lowered by this loss?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:30
What are the major products produced in the combustion of c10h22 under the following conditions? write balanced chemical equations for each. a. an excess of oxygen b. a slightly limited oxygen supply c. a very limited supply of oxygen d. the compound is burned in air
Answers: 2

Chemistry, 22.06.2019 07:30
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 12:00
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1

Chemistry, 22.06.2019 19:30
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2
You know the right answer?
The solubility of aspirin in water is 1 g per 300 mL at 25 degrees celsius. Assuming that your cryst...
Questions


History, 27.09.2019 20:40

Mathematics, 27.09.2019 20:40

History, 27.09.2019 20:40


Physics, 27.09.2019 20:40


Mathematics, 27.09.2019 20:40

Mathematics, 27.09.2019 20:40

Mathematics, 27.09.2019 20:40



History, 27.09.2019 20:40


Mathematics, 27.09.2019 20:40

English, 27.09.2019 20:40

Arts, 27.09.2019 20:40



Physics, 27.09.2019 20:40

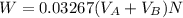
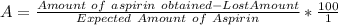

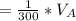
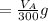
 to wash the crystallized aspirin then the lost during washing would be
to wash the crystallized aspirin then the lost during washing would be