Chemistry, 03.04.2020 06:06 seannalove6168
A sample of O2 of volume 4.56 L was collected over water at 25c and a total pressure of 1.00 atm. The partial pressure of water is 34.5 Torr. How many moles of O2molescules were collected?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 18:30
Calculate the change in entropy if br2(l) is converted into gaseous br atoms. s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 2


Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 3

Chemistry, 22.06.2019 21:30
Which of the following changes will decrease the total amount of gaseous solute able to be dissolved in a liter of liquid water? (2 points) decreasing temperature decreasing pressure decreasing surface area decreasing solute concentration
Answers: 1
You know the right answer?
A sample of O2 of volume 4.56 L was collected over water at 25c and a total pressure of 1.00 atm. Th...
Questions


English, 03.04.2020 03:30


Mathematics, 03.04.2020 03:30



History, 03.04.2020 03:30


Mathematics, 03.04.2020 03:30





Mathematics, 03.04.2020 03:31

Mathematics, 03.04.2020 03:31



Mathematics, 03.04.2020 03:31


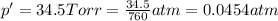
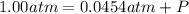
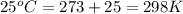
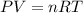 (ideal gas)
(ideal gas)