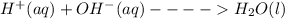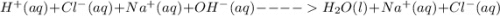Chemistry, 03.04.2020 02:44 josephvaldez518
HCl(aq)+NaOH(aq)→NaCl(aq)+H2O(l)
A student was given the task of titrating a 20.mL sample of 0.10MHCl(aq) with 0.10MNaOH(aq) . The HCl(aq) was placed in an Erlenmeyer flask. An equation for the reaction that occurs during the titration is given above.
(b) The equation above is not written in net ionic form. Write the correct net ionic equation for the reaction.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 17:10
Increasing the substrate concentration in an enzymatic reaction could overcome which of the following? a) the need for a coenzymeb) allosteric inhibitionc) competitive inhibitiond) insufficient cofactors
Answers: 1

Chemistry, 22.06.2019 21:00
As we move from left to right across the periodic table, what is the general trend? a) atomic radii increase. b) electronegavitiy decreases. c) nuclear shielding increases. d) metallic character decreases.
Answers: 1

Chemistry, 22.06.2019 21:00
Two nails have identical sizes and shapes. in one nail, 20 percent of the domains are lined up. in the other nail, 80 percent of the domains are lined up. which has stronger magnetic force? first answer gets brainliest!
Answers: 1

Chemistry, 23.06.2019 11:30
A) equal lines b) parallel lines c) perpendicular lines d) none of the above
Answers: 1
You know the right answer?
HCl(aq)+NaOH(aq)→NaCl(aq)+H2O(l)
A student was given the task of titrating a 20.mL samp...
A student was given the task of titrating a 20.mL samp...
Questions

Computers and Technology, 06.01.2020 20:31


Physics, 06.01.2020 20:31


Biology, 06.01.2020 20:31


Mathematics, 06.01.2020 20:31


Mathematics, 06.01.2020 20:31

Mathematics, 06.01.2020 20:31

English, 06.01.2020 20:31

Mathematics, 06.01.2020 20:31

Mathematics, 06.01.2020 20:31




Business, 06.01.2020 20:31


Engineering, 06.01.2020 20:31