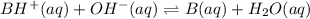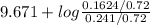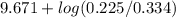Chemistry, 02.04.2020 02:30 dontcareanyonemo
A buffer that contains 0.17 M of a base, B and 0.39 M of its conjugate acid BH+, has a pH of 9.31. What is the pH after 0.02 mol of Ba(OH)2 are added to 0.72 L of the solution?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 01:30
An empty fuel tank can still contain and therefore can be even more dangerous than one full of liquid fuel.
Answers: 1

Chemistry, 22.06.2019 10:50
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1

Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2

Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2
You know the right answer?
A buffer that contains 0.17 M of a base, B and 0.39 M of its conjugate acid BH+, has a pH of 9.31. W...
Questions






History, 04.11.2020 18:10


Mathematics, 04.11.2020 18:10

English, 04.11.2020 18:10


Physics, 04.11.2020 18:10








Mathematics, 04.11.2020 18:10

History, 04.11.2020 18:10

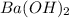 are added to 0.72 L of the solution is 9.5.
are added to 0.72 L of the solution is 9.5.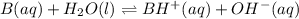
![pK_{a} + log \frac{[B]}{[BH^{+}]}](/tpl/images/0577/1202/d0a56.png)
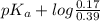
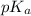 = 9.31 + 0.361
= 9.31 + 0.361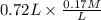
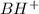 is as follows.
is as follows.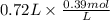
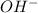 as follows.
as follows.