For the reaction H2(g) + I2(g) 2HI(g), Kc = 50.2 at 445ºC. If [H2] = [I2] = [HI] = 1.75 10-3 M at 445ºC, which one of these statements is true? A) The system is at equilibrium, thus no concentration changes will occur. B) The concentrations of HI and I2 will increase as the system approaches equilibrium. C) The concentration of HI will increase as the system approaches equilibrium. D) The concentrations of H2 and HI will fall as the system moves toward equilibrium. E) The concentrations of H2 and I2 will increase as the system approaches equilibrium

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:00
The pressure in a fluid is affected by which characteristics of that fluid
Answers: 1

Chemistry, 22.06.2019 21:00
In the experiment you asked to react hydrochloric acid and with sodium hydroxide. when measuring the volume of the reactants, which instrument would give the greatest precision.
Answers: 3

Chemistry, 23.06.2019 00:30
An ice cube with a volume of 45.0ml and a density of 0.9000g/cm3 floats in a liquid with a density of 1.36g/ml. what volume of the cube is submerged in the liquid
Answers: 3

You know the right answer?
For the reaction H2(g) + I2(g) 2HI(g), Kc = 50.2 at 445ºC. If [H2] = [I2] = [HI] = 1.75 10-3 M at...
Questions

Health, 07.07.2019 13:00

Mathematics, 07.07.2019 13:00

Mathematics, 07.07.2019 13:00

Mathematics, 07.07.2019 13:00





Mathematics, 07.07.2019 13:00

Mathematics, 07.07.2019 13:00

History, 07.07.2019 13:00


Mathematics, 07.07.2019 13:00




English, 07.07.2019 13:00

Mathematics, 07.07.2019 13:00

Mathematics, 07.07.2019 13:00

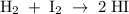
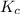
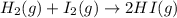
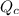 is written as:
is written as:![Q_c=\frac{[HI]^2}{[H_2]^1[I_2]^1}](/tpl/images/0575/8711/8578e.png)
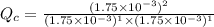
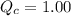
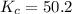
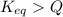 , the reaction will shift towards the right i.e. towards the product side.
, the reaction will shift towards the right i.e. towards the product side.