

Answers: 3
Another question on Chemistry



Chemistry, 22.06.2019 22:30
Molecular iodine, i2(g), dissociates into iodine atoms at 625 k with a first-order rate constant of 0.271 s−1. part a part complete what is the half-life for this reaction?
Answers: 3

Chemistry, 22.06.2019 23:00
What is the oxidation state of each individual carbon atom in c2o42−?
Answers: 1
You know the right answer?
The natural distribution of the isotopes of a hypothetical element is 60.795% at a mass of 281.99481...
Questions

English, 22.04.2020 18:43


Mathematics, 22.04.2020 18:43

History, 22.04.2020 18:43

Biology, 22.04.2020 18:44

Mathematics, 22.04.2020 18:44


Mathematics, 22.04.2020 18:44

History, 22.04.2020 18:44


Mathematics, 22.04.2020 18:44

Chemistry, 22.04.2020 18:44

English, 22.04.2020 18:45

Mathematics, 22.04.2020 18:45


Social Studies, 22.04.2020 18:45

English, 22.04.2020 18:45

Biology, 22.04.2020 18:45


English, 22.04.2020 18:45

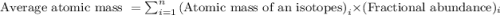 .....(1)
.....(1)![\text{Average atomic mass of element}=[(281.99481\times 0.60795)+(283.99570\times 0.22122)+(286.99423\times 0.17083)]\\\\\text{Average atomic mass of element}=283.291amu](/tpl/images/0572/8936/1f7da.png)


