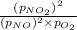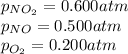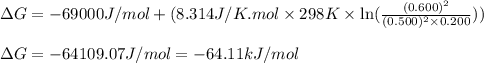For a gaseous reaction, standard conditions are 298 K and a partial pressure of 1 atm for all species. For the reaction 2 NO ( g ) + O 2 ( g ) − ⇀ ↽ − 2 NO 2 ( g ) the standard change in Gibbs free energy is Δ G ° = − 69.0 kJ/mol . What is ΔG for this reaction at 298 K when the partial pressures are P NO = 0.500 atm , P O 2 = 0.200 atm , and P NO 2 = 0.600 atm ?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Write the balanced equation for a reaction between aqueous nitric acid (hno3) and solid lithium metal (this is a single replacement reaction)
Answers: 1

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1


Chemistry, 22.06.2019 18:10
Areader can tell that the meaning of “obnoxious” will include “having the quality of something” because of the .a) prefix b)pronunciation c)suffix d) word root
Answers: 3
You know the right answer?
For a gaseous reaction, standard conditions are 298 K and a partial pressure of 1 atm for all specie...
Questions






Biology, 11.11.2020 22:30

Biology, 11.11.2020 22:30

English, 11.11.2020 22:30

English, 11.11.2020 22:30


Mathematics, 11.11.2020 22:30


Mathematics, 11.11.2020 22:30

Mathematics, 11.11.2020 22:30

English, 11.11.2020 22:30

Health, 11.11.2020 22:30

Biology, 11.11.2020 22:30

Mathematics, 11.11.2020 22:30


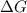 for the given reaction at 298 K is -64.11 kJ/mol
for the given reaction at 298 K is -64.11 kJ/mol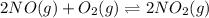
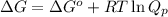
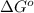 = Standard Gibbs free energy = -69.0 kJ/mol = -69000 J/mol (Conversion factor: 1 kJ = 1000 J)
= Standard Gibbs free energy = -69.0 kJ/mol = -69000 J/mol (Conversion factor: 1 kJ = 1000 J)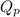 = Ratio of partial pressure of products and reactants =
= Ratio of partial pressure of products and reactants =