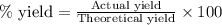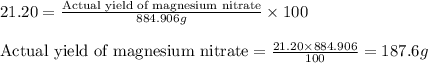Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:00
At 450 mm hg a gas has a volume of 760 l, what is its volume at standard pressure
Answers: 2

Chemistry, 22.06.2019 08:30
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Chemistry, 22.06.2019 12:00
Most materials are not magnetic because their magnetism has worn off. their magnetic domains are arranged randomly. they lack magnetic fields. earth’s heat has destroyed their magnetism.
Answers: 1
You know the right answer?
Assuming an efficiency of 21.20 % , calculate the actual yield of magnesium nitrate formed from 143....
Questions

English, 27.07.2020 07:01

Mathematics, 27.07.2020 07:01

Biology, 27.07.2020 07:01

Mathematics, 27.07.2020 07:01


Mathematics, 27.07.2020 07:01


Mathematics, 27.07.2020 07:01





Mathematics, 27.07.2020 07:01


Social Studies, 27.07.2020 07:01

World Languages, 27.07.2020 07:01


Medicine, 27.07.2020 07:01


Advanced Placement (AP), 27.07.2020 07:01

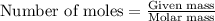 .....(1)
.....(1)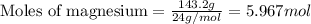
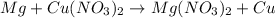
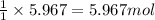 of magnesium nitrate
of magnesium nitrate