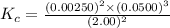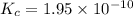Chemistry, 30.03.2020 23:54 dannaasc5475
1 Potassium chlorate decomposes to product potassium chloride and oxygen gas. 2KClO3(s) ⇔ 2KCl(s) + 3O2(g) When this reaction was run at room temperature, the following equilbrium concentrations were measured: [O2] = 0.0500 M; [KCl] = 0.00250 M; [KClO3] = 2.00 M What is the equilibrium constant for this reaction?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1

Chemistry, 23.06.2019 03:50
Which best describes the activation energy of a chemical reaction? a. the combined energy of all the reactants b. the amount of energy required for a reaction to occur c. the difference in energy between products and reactants d. the potential energy stored in the bonds of reactants and products
Answers: 1

Chemistry, 23.06.2019 09:30
What lessons does the history and study of the periodic table offer to other fields of science, and the pursuit science more generally
Answers: 3

Chemistry, 23.06.2019 13:30
Use the periodic table to classify each of the elements below. cadmium (cd): vanadium (v): xenon (xe): iodine (i): potassium (k): strontium (sr):
Answers: 3
You know the right answer?
1 Potassium chlorate decomposes to product potassium chloride and oxygen gas. 2KClO3(s) ⇔ 2KCl(s) +...
Questions

Mathematics, 03.04.2021 14:03

Health, 03.04.2021 14:03

Mathematics, 03.04.2021 14:03

Mathematics, 03.04.2021 14:03



English, 03.04.2021 14:03

Mathematics, 03.04.2021 14:03

Spanish, 03.04.2021 14:03

Mathematics, 03.04.2021 14:03







Social Studies, 03.04.2021 14:03

Computers and Technology, 03.04.2021 14:03

English, 03.04.2021 14:03

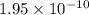
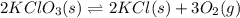
![K_c=\frac{[KCl]^2\times [O_2]^3}{[KClO_3]^2}](/tpl/images/0571/9600/641b5.png)