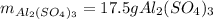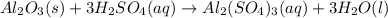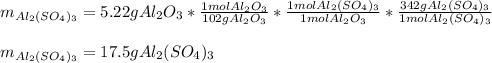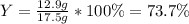Chemistry, 30.03.2020 23:55 guadalupemarlene2001
For the following reaction, 5.22 grams of aluminum oxide are mixed with excess sulfuric acid. The reaction yields 12.9 grams of aluminum sulfate. aluminum oxide (s) + sulfuric acid (aq) aluminum sulfate (aq) + water (l) What is the theoretical yield of aluminum sulfate ?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 16:00
If the same amount of cacl2 is added to equal volumes of water and maple syrup, which will have the higher temperature?
Answers: 1

Chemistry, 22.06.2019 09:00
Plz mark brainliest 30 points1) find the momentum of a 12 kg snowball that is rolling with a velocity of 9 m/s.2) an 8 ball with a mass of .5 kg is sitting at rest. it is hit by the cue ball (1 kg) traveling at 2.5 m/s. if the cue ball is at rest after the collision, how fast is the 8 ball traveling after the collision? 3) two football players are running toward each other. if the offensive player is 75 kg and is running 8 m/s, how fast must the 60 kg defensive player run in order for the two players to hit and stop?
Answers: 1

Chemistry, 22.06.2019 18:00
What amount of heat is exchanged when 106.2 grams of substance y goes from a liquid at 35 degrees celsius to a solid at the same temperature? melting point of substance y = 35 degrees c; δhvaporization = 3.67 j/mol; δhfusion = 3.30 j/mol. mwsubstance y = 28.22 g/mol. −12.4 j −3.51 x 102 j 1.24 x 101 j 351 j
Answers: 1

Chemistry, 22.06.2019 22:00
What mass of glucose is produced when 54g of water react with carbon dioxide
Answers: 1
You know the right answer?
For the following reaction, 5.22 grams of aluminum oxide are mixed with excess sulfuric acid. The re...
Questions

Mathematics, 04.11.2020 16:30

History, 04.11.2020 16:30


Arts, 04.11.2020 16:30


Mathematics, 04.11.2020 16:30

Advanced Placement (AP), 04.11.2020 16:30





Physics, 04.11.2020 16:30


Mathematics, 04.11.2020 16:30



History, 04.11.2020 16:30

Arts, 04.11.2020 16:30

English, 04.11.2020 16:30

Engineering, 04.11.2020 16:30