Chemistry, 30.03.2020 21:22 usagimiller
Coal gasification can be represented by the equation: 2 C(s) + 2 H2O(g) → CH4(g) + CO2(g) ΔH = ? Use the following information to find ΔH for the reaction above. CO(g) + H2(g) → C(s) + H2O(g) ΔH = -131 kJ CO(g) + H2O(g) → CO2(g) + H2(g) ΔH = -41 kJ CO(g) + 3 H2(g) → CH4(g) + H2O(g) ΔH = -206 kJ

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:30
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10^-3 m and k for the dissociation is 1.86x10^-5. ch3cooh(aq)+h2o(> h3o^+(aq)+ch3coo^-(aq)
Answers: 2

Chemistry, 22.06.2019 02:20
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3
You know the right answer?
Coal gasification can be represented by the equation: 2 C(s) + 2 H2O(g) → CH4(g) + CO2(g) ΔH = ? Use...
Questions


Mathematics, 26.09.2019 00:20


Mathematics, 26.09.2019 00:20

Geography, 26.09.2019 00:20


History, 26.09.2019 00:20


Social Studies, 26.09.2019 00:20


Social Studies, 26.09.2019 00:20

Social Studies, 26.09.2019 00:20

Social Studies, 26.09.2019 00:20

Social Studies, 26.09.2019 00:20


Social Studies, 26.09.2019 00:20

Social Studies, 26.09.2019 00:20

Social Studies, 26.09.2019 00:20

Social Studies, 26.09.2019 00:20

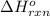 for the reaction is 15 kJ.
for the reaction is 15 kJ.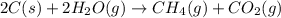
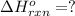

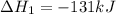 ( × 2)
( × 2)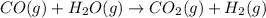

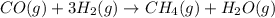
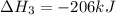
![\Delta H^o_{rxn}=[2\times (-\Delta H_1)]+[1\times \Delta H_2]+[1\times \Delta H_3]](/tpl/images/0571/2803/fd23d.png)
![\Delta H^o_{rxn}=[(2\times -(-131))+(1\times (-41))+(1\times (-206))]=15kJ](/tpl/images/0571/2803/ea721.png)