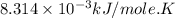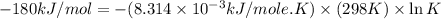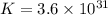Chemistry, 30.03.2020 21:03 manyah6189
Silver occurs in trace amounts in some ores of lead, and lead can displace silver from solution: Pb(s) + 2Ag+ (aq) LaTeX: \longrightarrow⟶ Pb2+(aq) + 2Ag(s) As a consequence, silver is a valuable byproduct in the industrial extraction of lead from its ores. Calculate K and LaTeX: \DeltaΔG° at 298K for this reaction. E°cell = .93 K: Enter as e notation to 1 decimal place (eg 1.2e3) LaTeX: \DeltaΔG°: Enter in kJ/mol to 0 decimal places. Use 96.5 kJ/Vmol e- for F (Faraday's constant). Do not use e notation.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Y=‐1x + 7 if y has a value of ‐24 what is the value of x?
Answers: 1

Chemistry, 21.06.2019 22:00
Which produce would best increase the amount of heat energy that is actually gained by calorimeter b
Answers: 1

Chemistry, 22.06.2019 02:20
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 04:50
Write the overall equation for the reaction for lithium battery
Answers: 2
You know the right answer?
Silver occurs in trace amounts in some ores of lead, and lead can displace silver from solution: Pb(...
Questions

Mathematics, 31.07.2021 09:50

Physics, 31.07.2021 09:50

Mathematics, 31.07.2021 09:50




Biology, 31.07.2021 09:50

Mathematics, 31.07.2021 09:50


History, 31.07.2021 09:50

Mathematics, 31.07.2021 09:50

Mathematics, 31.07.2021 09:50


Mathematics, 31.07.2021 09:50

Mathematics, 31.07.2021 09:50


English, 31.07.2021 09:50

Mathematics, 31.07.2021 09:50


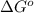 and K is, -180 kJ/mol and
and K is, -180 kJ/mol and 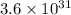
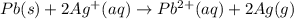
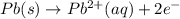
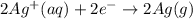
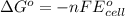
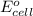 = standard electrode potential of the cell = 0.93 V
= standard electrode potential of the cell = 0.93 V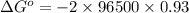
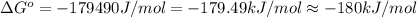
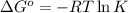
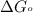 = standard Gibbs free energy = -180 kJ/mol
= standard Gibbs free energy = -180 kJ/mol