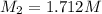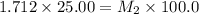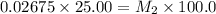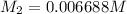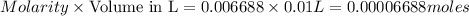Chemistry, 30.03.2020 21:08 studentms5313
Starting with a 6.847 M stock solution of HNO3, five standard solutions are prepared via serial dilution. At each stage, 25.00 mL of solution are diluted to 100.00 mL. Determine the concentration of and the number of moles of HNO3 in the final (most dilute, Md5) solution.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 10:10
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate,m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 2

Chemistry, 22.06.2019 18:30
Which of the following nuclei would be the least stable a 2 protons, 2 neutrons b 1 proton 1 neutron c 1 proton 3 neutrons d 1 proton 2 neutrons
Answers: 3

Chemistry, 22.06.2019 21:00
Write a balanced equation showing the formation of copper (ii) nitrite from its elements
Answers: 1
You know the right answer?
Starting with a 6.847 M stock solution of HNO3, five standard solutions are prepared via serial dilu...
Questions

History, 27.06.2019 23:30

Mathematics, 27.06.2019 23:30

Mathematics, 27.06.2019 23:30

Health, 27.06.2019 23:30

History, 27.06.2019 23:30


History, 27.06.2019 23:30


History, 27.06.2019 23:30

History, 27.06.2019 23:30



History, 27.06.2019 23:30



Advanced Placement (AP), 27.06.2019 23:30



Mathematics, 27.06.2019 23:30

Mathematics, 27.06.2019 23:30

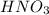 in the final solution is 0.006688 M and number of moles are 0.00006688
in the final solution is 0.006688 M and number of moles are 0.00006688
 = molarity of stock solution = 6.847 M
= molarity of stock solution = 6.847 M = volume of stock solution = 25.00 ml
= volume of stock solution = 25.00 ml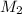 = molarity of ist dilute solution = ?
= molarity of ist dilute solution = ? = volume of first dilute solution = 100.0 ml
= volume of first dilute solution = 100.0 ml