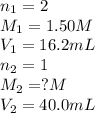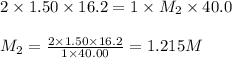Chemistry, 30.03.2020 21:17 nannagarvey9945
A volume of 40.0 mLmL of aqueous potassium hydroxide (KOHKOH) was titrated against a standard solution of sulfuric acid (H2SO4H2SO4). What was the molarity of the KOHKOH solution if 16.2 mLmL of 1.50 MM H2SO4H2SO4 was needed? The equation is 2KOH(aq)+H2SO4(aq)→K2SO4(aq)+2H2O(l )

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 12:30
Wind is related tot he movement or warm cold air masses which kind of heat transfer does this represent
Answers: 2

Chemistry, 22.06.2019 08:30
Which common material is an example of a polymer? (25 pts) a. steel b. plastic c. petroleum d. rubbing alcohol
Answers: 2

Chemistry, 22.06.2019 17:40
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3

Chemistry, 22.06.2019 22:00
Ill give u brainliest pls how is mass of carbon conserved during cellular respiration
Answers: 1
You know the right answer?
A volume of 40.0 mLmL of aqueous potassium hydroxide (KOHKOH) was titrated against a standard soluti...
Questions



Mathematics, 20.03.2022 09:00


Social Studies, 20.03.2022 09:00

Biology, 20.03.2022 09:00

Mathematics, 20.03.2022 09:00



English, 20.03.2022 09:10


Social Studies, 20.03.2022 09:10

Mathematics, 20.03.2022 09:10

English, 20.03.2022 09:10

Mathematics, 20.03.2022 09:10


Health, 20.03.2022 09:20


Mathematics, 20.03.2022 09:20

English, 20.03.2022 09:20

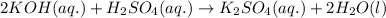
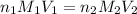
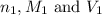 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 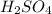
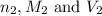 are the n-factor, molarity and volume of base which is KOH.
are the n-factor, molarity and volume of base which is KOH.