Chemistry, 30.03.2020 22:16 janahiac09
Part B When carbon is burned in air, it reacts with oxygen to form carbon dioxide. When 22.8 g of carbon were burned in the presence of 73.8 g of oxygen, 13.0 g of oxygen remained unreacted. What mass of carbon dioxide was produced? Express your answer to one decimal place and include the appropriate units. View Available Hint(s)

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 16:50
What is the composition, in atom percent, of an alloy that consists of 4.5 wt% pb and 95.5 wt% sn? the atomic weights for pb and sn are 207.19 g/mol and 118.71 g/mol, respectively.(a) 2.6 at% pb and 97.4 at% sn(b) 7.6 at% pb and 92.4 at% sn(c)97.4 at% pb and 2.6 at% sn(d) 92.4 at% pb and 7.6 at% sn
Answers: 2

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 2

Chemistry, 22.06.2019 17:30
Energy defines the different "states" of matter. in no more than 3 sentences, describe the amount of kinetic energy that each of the 3 states of matter possesses and relate that to the atom/molecular motion of each "state".
Answers: 2

Chemistry, 23.06.2019 00:00
Which is true about metals used for jewelry, such as platinum and gold? a. they have low flammability. b. they have low reactivity. c. they have high flammability. d. they have high reactivity.
Answers: 1
You know the right answer?
Part B When carbon is burned in air, it reacts with oxygen to form carbon dioxide. When 22.8 g of ca...
Questions




Mathematics, 22.07.2021 04:30


Mathematics, 22.07.2021 04:30


Physics, 22.07.2021 04:30

Physics, 22.07.2021 04:30






History, 22.07.2021 04:30



Mathematics, 22.07.2021 04:30

Mathematics, 22.07.2021 04:30

Social Studies, 22.07.2021 04:30

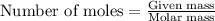 .....(1)
.....(1)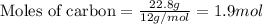
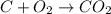
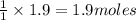 of carbon dioxide gas
of carbon dioxide gas