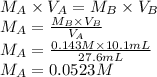Chemistry, 30.03.2020 19:38 cheaterman4121
A student is asked to standardize a solution of potassium hydroxide. He weighs out 1.08 g potassium hydrogen phthalate (KHC8H4O4, treat this as a monoprotic acid). It requires 36.8 mL of potassium hydroxide to reach the endpoint. A. What is the molarity of the potassium hydroxide solution? M This potassium hydroxide solution is then used to titrate an unknown solution of perchloric acid. B. If 10.1 mL of the potassium hydroxide solution is required to neutralize 27.6 mL of perchloric acid, what is the molarity of the perchloric acid solution? M

Answers: 3
Another question on Chemistry


Chemistry, 23.06.2019 09:30
Northern was a learned a fairly cold climate caused by see one from the atlantic ocean, but se was real and tends to be much warmer, sonia look good causes difference. a.cool wins cannot blow across a leg into the south mountains, b.prevent cold air from blowing over into south sea,c.the south is at much higher elevation, so it is closer to the sun, d.suppose early and has a sperience a drastic climate change in the past few years.
Answers: 1


You know the right answer?
A student is asked to standardize a solution of potassium hydroxide. He weighs out 1.08 g potassium...
Questions


History, 15.02.2020 19:20


Physics, 15.02.2020 19:21


Mathematics, 15.02.2020 19:22

Mathematics, 15.02.2020 19:22


Physics, 15.02.2020 19:22


Physics, 15.02.2020 19:23

History, 15.02.2020 19:24


Mathematics, 15.02.2020 19:27




Business, 15.02.2020 19:42