
Chemistry, 30.03.2020 17:30 johnsonkia873
Write chemical equations and corresponding equilibrium expressions for each of the two ionization steps of carbonic acid. Part A Write chemical equations for first ionization step of carbonic acid. Express your answer as a chemical equation. Identify all of the phases in your answer. nothing Request Answer Part B Complete previous part(s) Part C Write chemical equations for second ionization step of carbonic acid. Express your answer as a chemical equation. Identify all of the phases in your answer. nothing

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:50
If a reactant was removed, did the new equilibrium system shift to make more reactants or more products?
Answers: 1

Chemistry, 22.06.2019 08:30
How does the principle of electromagnetism explain the interaction between earth’s magnetic field and the solar wind?
Answers: 1

Chemistry, 22.06.2019 11:00
The human eye contains a molecule called 11-cis-retinal that changes shape when struck with light of sufficient energy. the change in shape triggers a series of events that results in an electrical signal being sent to the brain that results in vision. the minimum energy required to change the conformation of 11-cis-retinal within the eye is about 164 kj/mol.
Answers: 2

Chemistry, 23.06.2019 00:30
Gasoline has a density of 0.740 g/ml. if you have 328 grams of gasoline, what is the volume in milliliters?
Answers: 1
You know the right answer?
Write chemical equations and corresponding equilibrium expressions for each of the two ionization st...
Questions



History, 20.10.2020 18:01



Chemistry, 20.10.2020 18:01



Mathematics, 20.10.2020 18:01




English, 20.10.2020 18:01

English, 20.10.2020 18:01


Computers and Technology, 20.10.2020 18:01


Business, 20.10.2020 18:01

English, 20.10.2020 18:01

Mathematics, 20.10.2020 18:01

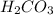 . It is a diprotic weak acid which means that it will release two hydrogen ions when dissolved in water
. It is a diprotic weak acid which means that it will release two hydrogen ions when dissolved in water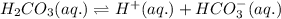
![Ka_1=\frac{[H^+][HCO_3^{-}]}{[H_2CO_3]}](/tpl/images/0570/5262/fb18f.png)
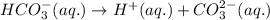
![Ka_2=\frac{[H^+][CO_3^{2-}]}{[HCO_3^-]}](/tpl/images/0570/5262/f465d.png)


